 During the 1960s—the early days of GC-MS analysis in chemistry and related disciplines—a large number of natural products, obtained from both biological and geological samples, were systematically analyzed. Among the studied samples were those that included polycyclic organic compounds such as optically active terpenoids. The analytical results were leading to interesting conclusions about fossil materials and their origin while the molecular structures of compounds from living organisms and sedimentary organic matter were compared. Knowledge of the molecular structure of steroids and terpenoids became detailed enough at that time to support such comparative analysis. At first, however, some confusion in terpenoid structure and nomenclature occurred [1]:
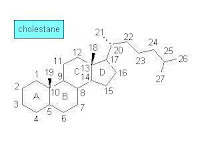
During the 1960s—the early days of GC-MS analysis in chemistry and related disciplines—a large number of natural products, obtained from both biological and geological samples, were systematically analyzed. Among the studied samples were those that included polycyclic organic compounds such as optically active terpenoids. The analytical results were leading to interesting conclusions about fossil materials and their origin while the molecular structures of compounds from living organisms and sedimentary organic matter were compared. Knowledge of the molecular structure of steroids and terpenoids became detailed enough at that time to support such comparative analysis. At first, however, some confusion in terpenoid structure and nomenclature occurred [1]:Many of the molecular structures that they [geochemists] puzzled out did indeed resemble the optically active cyclic terpenoids that the natural products chemists were collecting from organisms—except that most of these compounds in the oils and shales had been stripped of their double bonds and oxygen-containing functional groups, reduced, as it were, to their bare carbon skeletons. Excited to find what appeared to be molecular skeletons of known biological steroids and triterpenoids, the geochemists named the hydrocarbons accordingly, and sometimes misleading—precisely because they were missing some of the key information that distinguished the biological compounds. The sterane they called “ergostane” seemed to bear the carbon skeleton of the sterol ergosterol, which is made by fungi and was quite familiar to chemists. But ergosterol differs from brassicasterol only by a double bond in the ring structure, which, of course, was missing from the steranes in the rocks, and the relatively large quantities of ergostane that the geochemists found in in rocks and oils probably had little to do with fungi and everything to do with a certain very productive group of unicellular algae. Likewise, sitosterol, which is plentiful in land plants, and stigmasterol, which is abundant in land plants and algae, are distinguished by an extra double bond in stigmasterol, so one really can't tell whether the 29-carbon sterane that geochemists first called sitostane and later stigmastane derives from land plants or algae or both. Eventually, when the geochemists gave up on trying to distinguish the orientation of the side groups in the fossil molecules, they took to calling them simply 24-methylcholestane and 24-ethylcholestane.The above sketch of the molecular structure of cholestane (C27H48) shows the carbon numbering and ring lettering used in steranes.
Keywords: paleontology, petroleum chemistry, hydrocarbons, steroids, terpenoids, steranes, alkyl-substituted cholestanes, molecular similarity, molecular difference, molecular side chains, functional groups
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; pp. 56-57.



No comments:
Post a Comment